Dosing and Administration
A regimen of a tezacaftor/ivacaftor tablet taken in the morning and an ivacaftor tablet taken in the evening, approximately 12 hours apart1
- SYMDEKO should always be taken with fat-containing food to ensure adequate absorption. Examples of meals or snacks that contain fat are those prepared with butter or oils or those containing eggs, cheeses, nuts, whole milk, or meats
- Food or drink containing grapefruit should be avoided during treatment with SYMDEKO
-
Patients should continue taking their other CF therapies as prescribed
| ← ~12 hours apart → | ||
| Patients age 6 through 11 years, weighing <30 kg | ||
| Recommended dose | ivacaftor 75 mg |
ivacaftor 75 mg
|
| Patients age 6 through 11 years, weighing ≥30 kg and patients 12 years and older | ||
| Recommended dose | ivacaftor 150 mg |
ivacaftor 150 mg
|
| Tablets are not actual size. | ||
← ~12 hours apart →
Patients age 6 through 11 years, weighing <30 kg
Recommended dose
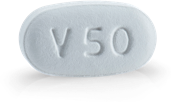
tezacaftor 50 mg
ivacaftor 75 mg

ivacaftor 75 mg
Patients age 6 through 11 years, weighing ≥30 kg and patients 12 years and older
Recommended dose

tezacaftor 100 mg
ivacaftor 150 mg

ivacaftor 150 mg
Tablets are not actual size
← ~12 hours apart →
Patients age 6 through 11 years, weighing <30 kg
Recommended dose

tezacaftor 50 mg
ivacaftor 75 mg

ivacaftor 75 mg
Patients age 6 through 11 years, weighing ≥30 kg and patients 12 years and older
Recommended dose

tezacaftor 100 mg
ivacaftor 150 mg

ivacaftor 150 mg
Tablets are not actual size.
| ← ~12 hours apart → | ||
| Recommended dose adjustments for hepatic impairmenta | ||
| Severe hepatic impairment (Child-Pugh Class C)b |
USE WITH CAUTION OR LESS FREQUENTLYc tezacaftor/ivacaftor dose |
No ivacaftor dose |
| Moderate hepatic impairment (Child-Pugh Class B) |
tezacaftor/ivacaftor dose | No ivacaftor dose |
| Recommended dose adjustments for concomitant use with CYP3A inducers or inhibitorsd | ||
| Strong CYP3A inducers | concomitant use not recommended |
concomitant use not recommended |
| Strong CYP3A inhibitors |
Approximately 3-4 days apart (twice in a week) tezacaftor/ivacaftor dose |
No ivacaftor dose |
| Moderate CYP3A inhibitors |
Alternate tablets every daye DAY 1 tezacaftor/
DAY 2 |
No ivacaftor dose |
← ~12 hours apart →
Recommended dose adjustments for hepatic impairmenta
Severe hepatic impairment
(Child-Pugh Class C)b
USE WITH CAUTION OR LESS FREQUENTLYC
tezacaftor/ivacaftor dose
No ivacaftor dose
Moderate hepatic impairment
(Child-Pugh Class B)
tezacaftor/ivacaftor dose
No ivacaftor dose
Recommended dose adjustments for concomitant use with CYP3A inducers or inhibitorsd
Strong CYP3A inducers
CONCOMITANT USE
NOT RECOMMENDED
CONCOMITANT USE
NOT RECOMMENDED
Strong CYP3A inhibitors
APPROXIMATELY 3-4 DAYS APART (TWICE IN A WEEK)
tezacaftor/ivacaftor dose
No ivacaftor dose
Moderate CYP3A inhibitors
ALTERNATE TABLETS EVERY DAYe
- DAY 1 tezacaftor/
ivacaftor dose - DAY 2
ivacaftor dose
No ivacaftor dose
← ~12 hours apart →
Recommended dose adjustments for hepatic impairmenta
Severe hepatic impairment
(Child-Pugh Class C)b
USE WITH CAUTION OR LESS FREQUENTLYC
tezacaftor/ivacaftor dose
No ivacaftor dose
Moderate hepatic impairment
(Child-Pugh Class B)
tezacaftor/ivacaftor dose
No ivacaftor dose
Recommended dose adjustments for concomitant use with CYP3A inducers or inhibitorsd
Strong CYP3A inducers
CONCOMITANT USE
NOT RECOMMENDED
CONCOMITANT USE
NOT RECOMMENDED
Strong CYP3A inhibitors
APPROXIMATELY 3-4 DAYS APART (TWICE IN A WEEK)
tezacaftor/ivacaftor dose
No ivacaftor dose
Moderate CYP3A inhibitors
ALTERNATE TABLETS EVERY DAYe
- DAY 1 tezacaftor/
ivacaftor dose - DAY 2
ivacaftor dose
No ivacaftor dose
aNo dose adjustments are necessary for mild hepatic impairment.1
bStudies have not been conducted in patients with severe hepatic impairment.1
cUse with caution at an adjusted dose after weighing the risks and benefits of treatment.1
dNo dose adjustment necessary for mild/moderate CYP3A inducers or mild CYP3A inhibitors.1
eContinue to take alternate tablets every day.1
- SYMDEKO has not been studied in patients with moderate or severe renal impairment or in patients with end-stage renal disease. No dose adjustment is recommended for mild and moderate renal impairment. Caution is recommended in patients with severe renal impairment or end-stage renal disease
- The safety and efficacy of SYMDEKO in patients with CF younger than 6 years of age have not been studied
Missed Dose1
- If 6 hours or less have passed since the missed morning or evening dose, the patient should take the missed dose as soon as possible and continue on the original schedule
- If more than 6 hours have passed since the missed morning or evening dose, the patient should not take the missed dose
- The next scheduled dose can be taken at the usual time. More than one dose should not be taken at the same time
SYMDEKO should always be taken with fat-containing foods to ensure adequate absorption1
Examples of Fat-Containing Foods1
Fat-containing foods help the body absorb SYMDEKO. A typical CF diet will satisfy this requirement. When counseling patients, the list below provides examples of a variety of fat-containing foods to take with SYMDEKO.
- Cheese pizza
- Eggs
- Peanut butter
- Whole milk
- Whole-milk cheese
- Whole-milk yogurt
| Child-Pugh Classification System2 | ||||
|---|---|---|---|---|
| Factor | Units | 1 Point | 2 Points | 3 Points |
| Serum bilirubin | µmol/L mg/dL |
<34 <2.0 |
34-51 2.0-3.0 |
>51 >3.0 |
| Serum albumin | g/dL | >3.5 | 3.0-3.5 | <3.0 |
| Prothrombin time | Seconds prolonged INR |
0-4 <1.7 |
4-6 1.7-2.3 |
>6 >2.3 |
| Ascites | N/A | None | Easily controlled | Poorly controlled |
| Hepatic encephalopathy | N/A | None | Minimal | Advanced |
| Child-Pugh Classification System2 | ||||
|---|---|---|---|---|
| Serum bilirubin | ||||
| Units | 1 Point | 2 Points | 3 Points | |
| µ mol/L mg/dL | <34 <2.0 | 34-51 2.0-3.0 | >51 >3.0 | |
| Serum albumin | ||||
| Units | 1 Point | 2 Points | 3 Points | |
| g/dL | >3.5 | 3.0-3.5 | <3.0 | |
| Prothrombin time | ||||
| Units | 1 Point | 2 Points | 3 Points | |
| Seconds prolonged INR | 0-4 <1.7 | 4-6 1.7-2.3 | >6 >2.3 | |
| Ascites | ||||
| Units | 1 Point | 2 Points | 3 Points | |
| N/A | None | Easily controlled | Poorly controlled | |
| Hepatic encephalopathy | ||||
| Units | 1 Point | 2 Points | 3 Points | |
| N/A | None | Minimal | Advanced | |
- The Child-Pugh classification system is used to assess the severity of liver disease. The Child-Pugh score is calculated by adding the scores of the 5 factors listed above. The patient receives a score of 1, 2, or 3 for each factor, which are added together to get the total Child-Pugh score
- According to this system, there are 3 levels of hepatic impairment—mild (Class A, score 5 or 6), moderate (Class B, score 7 to 9), and severe (Class C, score 10-15)
INR, international normalized ratio; N/A, not applicable
INDICATIONS AND USAGE
SYMDEKO is indicated for the treatment of cystic fibrosis (CF) in patients age 6 years and older who are homozygous for the F508del mutation or who have at least one mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that is responsive to tezacaftor/ivacaftor based on in vitro data and/or clinical evidence.
If the patient’s genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of a CFTR mutation followed by verification with bi-directional sequencing when recommended by the mutation test instructions for use.
INDICATIONS AND USAGE
SYMDEKO is indicated for the treatment of cystic fibrosis (CF) in patients age 6 years and older who are homozygous for the F508del mutation or who have at least one mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that is responsive to tezacaftor/ivacaftor based on in vitro data and/or clinical evidence.
If the patient’s genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of a CFTR mutation followed by verification with bi-directional sequencing when recommended by the mutation test instructions for use.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Transaminase (ALT or AST) Elevations
- Elevated transaminases have been observed in patients with CF receiving SYMDEKO, as well as with ivacaftor monotherapy. Assessments of transaminases (ALT and AST) are recommended prior to initiating SYMDEKO, every 3 months during the first year of treatment, and annually thereafter. For patients with a history of transaminase elevations, more frequent monitoring should be considered
- Dosing should be interrupted in patients with significant elevations of transaminases (e.g., ALT or AST >5x upper limit of normal [ULN], or ALT or AST >3x ULN with bilirubin >2x ULN) and laboratory tests should be closely followed until abnormalities resolve. Following resolution of transaminase elevations, consider the benefits and risks of resuming treatment
Hypersensitivity Reactions, Including Anaphylaxis
- Hypersensitivity reactions, including cases of anaphylaxis, have been reported in the postmarketing setting. If signs or symptoms of serious hypersensitivity reactions develop during treatment, discontinue SYMDEKO and institute appropriate therapy. Consider the benefits and risks for the individual patient to determine whether to resume treatment with SYMDEKO
Concomitant Use With CYP3A Inducers
- Exposure to ivacaftor is significantly decreased and exposure to tezacaftor may be reduced by concomitant use of CYP3A inducers, which may reduce the therapeutic effectiveness of SYMDEKO. Co-administration of SYMDEKO with strong CYP3A inducers, such as rifampin, rifabutin, phenobarbital, carbamazepine, phenytoin, and St. John’s wort, is not recommended
Cataracts
- Cases of non-congenital lens opacities have been reported in pediatric patients treated with SYMDEKO, as well as with ivacaftor monotherapy. Baseline and follow-up ophthalmological examinations are recommended in pediatric patients initiating treatment with SYMDEKO
ADVERSE REACTIONS
Serious Adverse Reactions
- Serious adverse reactions, whether considered drug-related or not by the investigators, that occurred more frequently in patients treated with SYMDEKO compared to placebo included distal intestinal obstruction syndrome, 3 (0.6%) patients treated with SYMDEKO vs. 0 placebo patients
Most Common Adverse Reactions
- The most common adverse reactions in Trials 1 and 3 occurring in ≥3% of patients treated with SYMDEKO (N=334) and at a higher rate than for placebo (N=343) were headache, nausea, sinus congestion, and dizziness
- The safety profile in patients age 6 to less than 12 years from an open-label Phase 3 trial (N=70) was similar to that observed in Trials 1 and 3
USE IN SPECIFIC POPULATION
Pediatric Use
- The safety and effectiveness of SYMDEKO in patients with CF younger than 6 years of age have not been studied
Click here to access full Prescribing Information.
References:
1. SYMDEKO [prescribing information]. Boston, MA: Vertex Pharmaceuticals Incorporated; August 2023. 2. Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Manual of Medicine. 17th ed. New York, NY: McGraw-Hill Medical; 2009.
INDICATIONS AND USAGE
SYMDEKO is indicated for the treatment of cystic fibrosis (CF) in patients age 6 years and older who are homozygous for the F508del mutation or who have at least one mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that is responsive to tezacaftor/ivacaftor based on in vitro data and/or clinical evidence.
If the patient’s genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of a CFTR mutation followed by verification with bi-directional sequencing when recommended by the mutation test instructions for use.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Transaminase (ALT or AST) Elevations
- Elevated transaminases have been observed in patients with CF receiving SYMDEKO, as well as with ivacaftor monotherapy. Assessments of transaminases (ALT and AST) are recommended prior to initiating SYMDEKO, every 3 months during the first year of treatment, and annually thereafter. For patients with a history of transaminase elevations, more frequent monitoring should be considered
- Dosing should be interrupted in patients with significant elevations of transaminases (e.g., ALT or AST >5x upper limit of normal [ULN], or ALT or AST >3x ULN with bilirubin >2x ULN) and laboratory tests should be closely followed until abnormalities resolve. Following resolution of transaminase elevations, consider the benefits and risks of resuming treatment
Hypersensitivity Reactions, Including Anaphylaxis
- Hypersensitivity reactions, including cases of anaphylaxis, have been reported in the postmarketing setting. If signs or symptoms of serious hypersensitivity reactions develop during treatment, discontinue SYMDEKO and institute appropriate therapy. Consider the benefits and risks for the individual patient to determine whether to resume treatment with SYMDEKO
Concomitant Use With CYP3A Inducers
- Exposure to ivacaftor is significantly decreased and exposure to tezacaftor may be reduced by concomitant use of CYP3A inducers, which may reduce the therapeutic effectiveness of SYMDEKO. Co-administration of SYMDEKO with strong CYP3A inducers, such as rifampin, rifabutin, phenobarbital, carbamazepine, phenytoin, and St. John’s wort, is not recommended
Cataracts
- Cases of non-congenital lens opacities have been reported in pediatric patients treated with SYMDEKO, as well as with ivacaftor monotherapy. Baseline and follow-up ophthalmological examinations are recommended in pediatric patients initiating treatment with SYMDEKO
ADVERSE REACTIONS
Serious Adverse Reactions
- Serious adverse reactions, whether considered drug-related or not by the investigators, that occurred more frequently in patients treated with SYMDEKO compared to placebo included distal intestinal obstruction syndrome, 3 (0.6%) patients treated with SYMDEKO vs. 0 placebo patients
Most Common Adverse Reactions
- The most common adverse reactions in Trials 1 and 3 occurring in ≥3% of patients treated with SYMDEKO (N=334) and at a higher rate than for placebo (N=343) were headache, nausea, sinus congestion, and dizziness
- The safety profile in patients age 6 to less than 12 years from an open-label Phase 3 trial (N=70) was similar to that observed in Trials 1 and 3
USE IN SPECIFIC POPULATIONS
Pediatric Use
- The safety and effectiveness of SYMDEKO in patients with CF younger than 6 years of age have not been studied
Click here to access full Prescribing Information.
References:
1. SYMDEKO [prescribing information]. Boston, MA: Vertex Pharmaceuticals Incorporated; August 2023. 2. Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Manual of Medicine. 17th ed. New York, NY: McGraw-Hill Medical; 2009.
